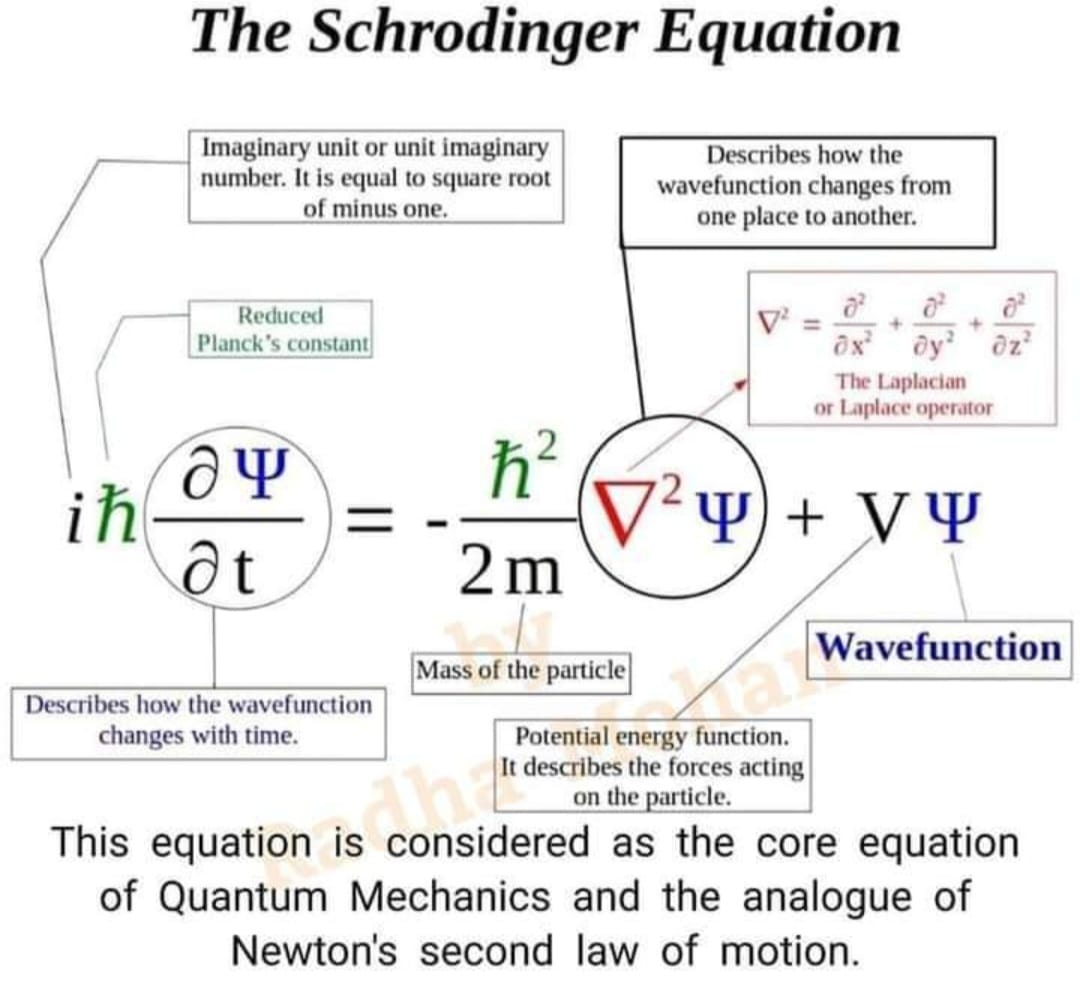
The Schrodinger equation was formulated in 1926 by Austrian physicist Erwin Schrodinger. Used in physics, specifically quantum mechanics, it is an equation that describes how the quantum state of a physical system changes in time.
In the standard interpretation of quantum mechanics, the quantum state, also called a wavefunction or state vector, is the most complete description that can be given to a physical system. Solutions to Schrodinger's equation describe not only molecular, atomic and subatomic systems, but also macroscopic systems, possibly even the whole universe.
The most general form is the time-dependent Schrodinger equation, which gives a description of a system evolving with time. For systems in a stationary state (i.e., where the Hamiltonian is not explicitly dependent on time), the time-independent Schrodinger equation is sufficient. Approximate solutions to the time-independent Schrodinger equation are commonly used to calculate the energy levels and other properties of atoms and molecules.
Schrodinger's equation can be mathematically transformed into Werner Heisenberg's matrix mechanics, and into Richard Feynman's path integral formulation. The Schrodinger equation describes time in a way that is inconvenient for relativistic theories, a problem which is not as severe in matrix mechanics and completely absent in the path integral formulation. wikipedia - Schrodinger equation
Erwin Schrödinger, one of the founding figures of quantum mechanics, had a complex and evolving perspective on the nature of the wave function (often denoted by the Greek letter Ψ). His views were deeply influenced by his work on wave mechanics and his philosophical inclinations. Here's a detailed look at Schrödinger's beliefs about the reality of the wave function:
1. Schrödinger's Wave Mechanics
- In 1926, Schrödinger formulated wave mechanics, which describes the behavior of quantum systems using a wave function Ψ.
- The Schrödinger equation, a cornerstone of quantum mechanics, governs how the wave function evolves over time.
- Initially, Schrödinger hoped that the wave function could represent a real, physical wave, akin to classical waves (e.g., sound or water waves).
2. Early Belief in a Real Wave
- In the early stages of developing wave mechanics, Schrödinger believed that the wave function might correspond to a physical reality, such as a matter wave or a charge density wave.
- He was inspired by Louis de Broglie's idea of matter waves and sought to interpret the wave function as a real, continuous entity in space and time.
3. Challenges to the Real Wave Interpretation
- Schrödinger's initial hope for a classical-like wave interpretation faced significant challenges:
- The wave function is a complex-valued function, making it difficult to interpret as a physical wave in the classical sense.
- The probabilistic interpretation of the wave function, championed by Max Born, suggested that |Ψ|² represents the probability density of finding a particle in a given state, rather than a physical wave.
- The wave function for multi-particle systems exists in a high-dimensional configuration space, not in ordinary 3D space, further complicating a classical wave interpretation.
4. Schrödinger's Later Views
- Over time, Schrödinger became more skeptical of the idea that the wave function represents a real, physical wave.
- He struggled with the implications of quantum mechanics, particularly the role of the observer and the collapse of the wave function.
- Schrödinger was critical of the Copenhagen interpretation, which treats the wave function as a mathematical tool for calculating probabilities rather than a physical entity.
5. Schrödinger's Cat Paradox
- In 1935, Schrödinger proposed his famous "cat paradox" to highlight the absurdity of applying quantum mechanics to macroscopic systems.
- The paradox illustrates the strange implications of the Copenhagen interpretation, where a cat in a sealed box could be considered simultaneously alive and dead until observed.
- This thought experiment reflects Schrödinger's discomfort with the idea that the wave function's collapse depends on observation, rather than representing an objective reality.
6. Philosophical Inclinations
- Schrödinger was influenced by Eastern philosophy and the idea of unity in nature, which may have shaped his views on the wave function.
- He leaned toward a more holistic interpretation of quantum mechanics, where the wave function might represent a deeper, underlying reality, though not necessarily a classical wave.
Conclusion
Schrödinger initially believed in the possibility of interpreting the wave function as a real, physical wave, but he later became more skeptical due to the challenges posed by quantum mechanics. While he never fully abandoned the idea of a deeper reality underlying the wave function, he recognized the limitations of interpreting it as a classical wave. His work and philosophical reflections continue to influence discussions about the interpretation of quantum mechanics and the nature of the wave function. [From DeepSeek]
See Also
Born Oppenheimer approximation
Equation
equation of forces
Figure 14.12 - Triple Equations to Represent a Single Sympathetic Event
magnetic moment
Maxwell Equations
On the Partial Differential Equations of Mathematical Physics
Eighteen Attributes or Dimensions
Renner-Teller Effect
Rotational-vibrational coupling
spin-orbit coupling
spintronics
Sympathetic Oscillation
Sympathetic Vibration
Sympathy
Wave
wavefunction
3.23 - Hydrodynamic Equations - Vortex Motions
9.30 - Eighteen Attributes of a Wave
12.11 - Eighteen Attributes or Dimensions
14.36 - Triple Equations
