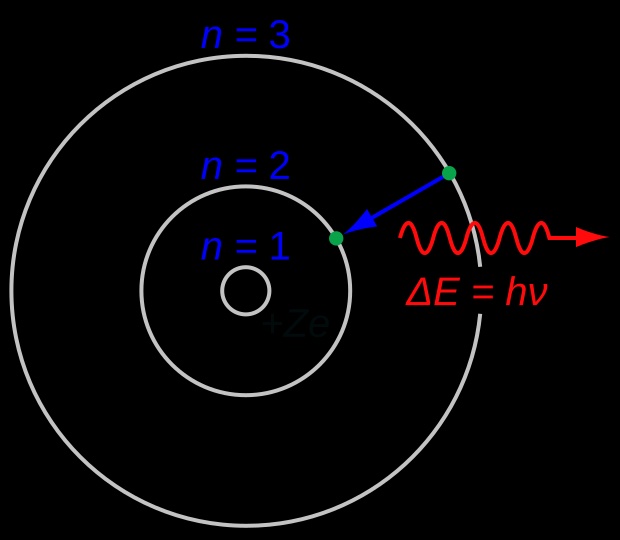
Bohr Model
In atomic physics, the Bohr model or Rutherford–Bohr model was the first successful model of the atom. Developed from 1911 to 1918 by Niels Bohr and building on Ernest Rutherford's nuclear model, it supplanted the plum pudding model of J J Thomson only to be replaced by the quantum atomic model in the 1920s. It consists of a small, dense nucleus surrounded by orbiting electrons. It is analogous to the structure of the Solar System, but with attraction provided by electrostatic force rather than gravity, and with the electron energies quantized (assuming only discrete values). wikipedia
ChatGPT compares the Bohr Model with SVP [12/26/24]: https://chatgpt.com/share/676d4bd2-a748-800d-9b9c-e8c54c96b975
See Also
